18
Jahre der Exzellenz
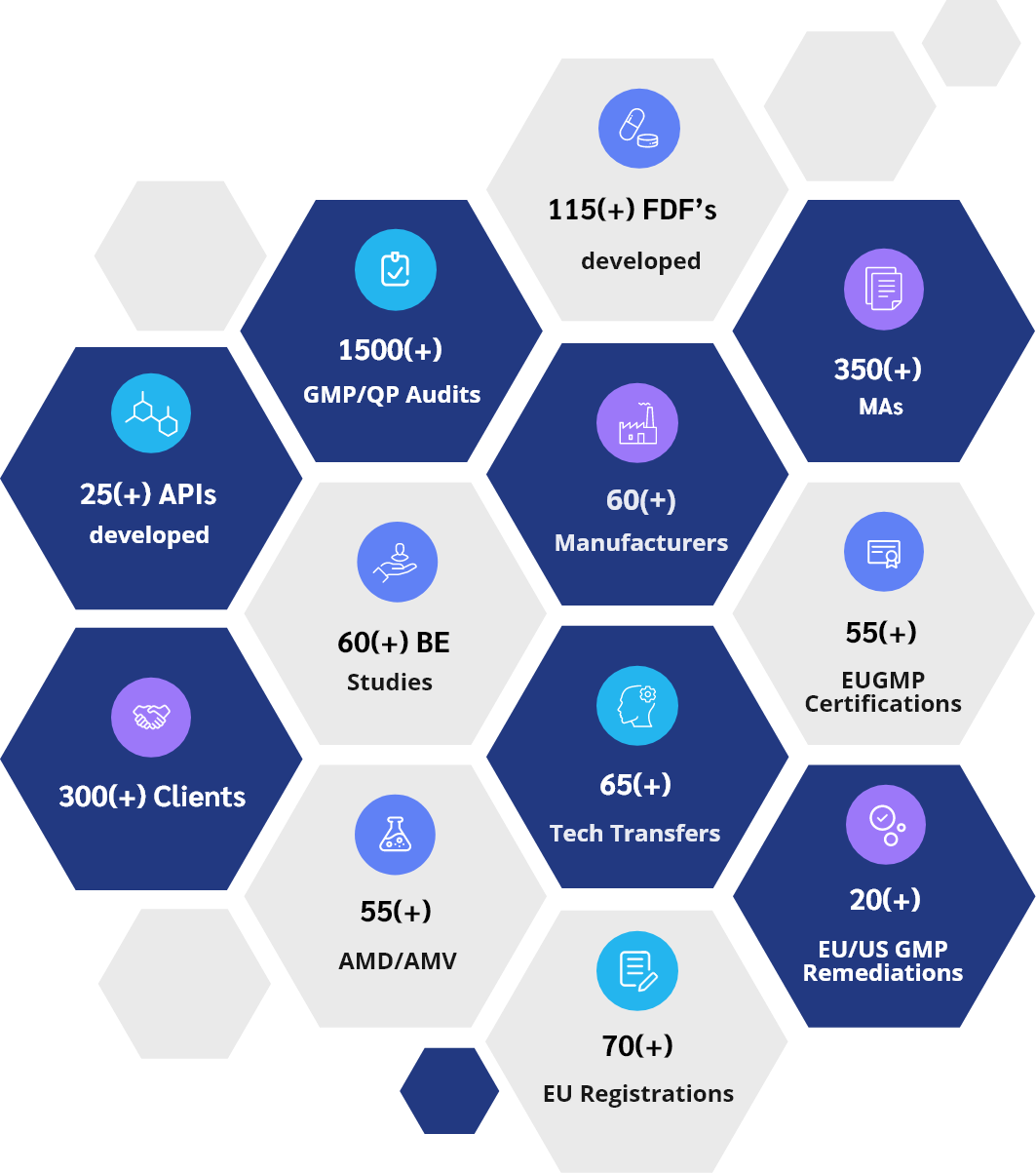
PharmSol wurde 2004 gegründet und hat sich zu einem globalen Pharmaunternehmen entwickelt, das intelligente Produkte und maßgeschneiderte Lösungen für die ganze Welt anbietet. PharmSol´s Erfolgsbilanz ist auf die umfassende technische Kompetenz seines Teams, sein Bestreben, die erste Wahl für seine Kunden zu sein, seinen Fokus auf den Aufbau langfristiger Beziehungen und sein langjähriges Engagement für den Fortschritt der pharmazeutischen Industrie und der Gemeinschaft zurückzuführen.
Seit seiner Gründung vor 18 Jahren hat PharmSol einen Weg des ständigen Wachstums beschritten und dabei nicht nur seine globale Reichweite erweitert, sondern auch die von uns angebotenen Produkte und Lösungen, das vielfältige und doch detaillierte Fachwissen unseres Teams und die Anzahl der Kunden, deren Erwartungen wir übertroffen haben.
Heute hat PharmSol weltweit einen Ruf erworben, indem sie viele “erste Anmelder”-Produkte entwickelt und registriert und rückwärts integriert hat. Als Ergebnis besitzen wir ein umfangreiches Portfolio an Produkten und Technologien, die entweder in-house entwickelt wurden oder in Zusammenarbeit mit anderen Unternehmen entstanden sind. Wir sind bestrebt, neue Produkte auf den Markt zu bringen und neue Ideen und Technologien umzusetzen, um den Anforderungen der Zukunft gerecht zu werden.

Jahre der Exzellenz

Erfolgreiche Produkte und Technologien

Erfolgreiche Lösungen
PharmSol ist dafür bekannt, dass es integrierte Lösungen anbietet, die allen seinen Kunden einen Zugang aus einer Hand ermöglichen und eine nahtlose und optimale Erfüllung aller Aufgaben gewährleisten. Auf den wichtigsten Märkten und dank unserer Erfahrung und operativen Flexibilität können wir die Herausforderungen unserer Kunden in den Bereichen pharmazeutische Vorschriften, Produktentwicklung, Registrierung, Technik, Marktangelegenheiten und Lieferung von Produkten bewältigen.
PharmSol hat seine Nische erweitert, indem es ergänzend oder umfangreich maßgeschneiderte Lösungen zur Erfüllung der Anforderungen in den Bereichen Produktentwicklung, IPR, Facility Design, Compliance, Registrierungen, Marktzugang, Audits und Lieferkette anbietet, wobei der Schwerpunkt auf den Anforderungen der EU, der USA, der TGA, der WHO und anderer internationaler Regulierungsbehörden liegt.
PharmSol strebt weiterhin nach Spitzenleistungen und erreicht sein Ziel, für immer der Marktführer in der Pharmabranche und die erste Wahl für seine Kunden zu sein. Die visionäre Führung von PharmSol, die auf fast 40 Jahre Erfolg zurückblicken kann, setzt weiterhin auf Innovation und Wachstum, indem sie ihre Präsenz in Afrika und Fernostasien festigt und neue Unternehmen wie das Eminus eGlobal Institute gründet, die dazu bestimmt sind, die Pharmabranche zu verändern.

Wir haben uns dem Streben nach Spitzenleistungen durch intelligente und hochwertige Produkte und die Bereitstellung von Expertenlösungen für die Bedürfnisse von Pharma und Biotech verschrieben.

Wir wollen einer der weltweit führenden und zuverlässigsten Partner für Produkte und Lösungen sein.

Managing Director
Sunil founded PharmSol in 2004 and led the company as Managing Director. His C-level substantial expertise in all facets of Pharmaceutical Business, Diversity Management, International Business, Mergers & Acquisitions and Strategic Alliances brought PharmSol to operational level of excellence and growth. A Chemical Engineer by qualification, with over 36 years of leadership experience in large pharma and established track record of entrepreneurial success within the pharmaceutical industry. Prior to founding PharmSol, he served for 20 years in various MNCs of repute. He has designed and mentored several green field projects for APIs and FDFs, all of which have been USFDA and/or EU certified.

Executive Director
Lakshmi is the Executive Director of PharmSol Group, having responsibility for the company’s strategy function and stakeholder relationships including clients’ relations, and corporate responsibility. He has over 26 years of diversified expertise and operational experience in Regulatory Affairs, QA, IPR, Bulk Drugs, Formulations and Fine Chemical Industries. Prior to his current role, Lakshmi worked in various Indian MNCs and International Companies. Lakshmi is a Pharmaceutical Technologist with Post Graduate Diploma in Patent Law and an MBA in Operations, International Business. His professional accreditations include APIC Certified Auditor, Associate Member of European QP Association, ECA Certified Computer Validation Manager, formerly certified for Temperature - Controlled Cargo Operations and CEIV Pharma Independent Validator of IATA.

Senior Business Associate
Christian has been a Director of PharmSol since inception. He is an entrepreneur with extensive experience in the pharmaceutical & allied fields, has been a guiding brain behind foundation of several businesses in Europe. At PharmSol, he has been supporting the strategy and business development functions. He is a graduate in logistic operations and has transitioned into pharmaceuticals early in his career. His skills in leadership and expertise in assembling people together, while achieving essential aspirations have led to multiple achievements throughout the years.

Director – Strategic Business
Mr. Arun holds a BE (Hons) Degree in Mechanical Engineering from BITS Pilani and a Post Graduate Diploma in Management Studies from JBIMS, University of Mumbai with over 35 years of professional experience. He specializes in international market development in industries related to chemicals, colors, technology, pharmaceuticals, and Agro products. He is also a faculty member in various reputed MBA institutions such as ICT Mumbai, JBIMS, World Trade Institute, and SP Jain Global Management Institute in Mumbai – with a teaching experience of over 22 years. He also works as a consultant to new Indian start-ups in the areas of business building since 2015.

Senior Scientific Adviser
Borut holds a Post Doctorate in molecular biology and has a rich experience of over 35 years into the field of Biosimilar, Nano Technology, Biochemistry and Biotechnology. He was one of the key members of the committee on drafting the EU Guidelines on Biosimilar and was also appointed as an expert in European Pharmacopeia for biological pharmaceuticals as well as at the European Agency for Medicines in London (EMEA). Borut co-authored several patent applications and has written over 180 scientific and professional articles which were published in more than 100 leading scientific journals. Currently, he is heading the Biotechnology Unit and is a full-time professor at the Faculty of Pharmacy of the University of Ljubljana. He is also a part of Editorial Board of the official journal of Slovenian Pharmaceutical Society and International Journal of Medicinal Mushrooms. As a Senior Scientific Adviser of PharmSol, he directs the PharmSol Team that focuses on the development, compliance, and regulatory aspects of biopharmaceutical projects.

Senior Business Associate
Andrej is a US based Pharmacist and Entrepreneur with a range of global experience. He brings to PharmSol a rich global experience of over 25 years within the areas of product development, cross licensing, technology transfers and strategic alliances negotiations and other related operations within the pharmaceutical industry. He holds an Executive MBA degree in Pharmaceutical Marketing and has been supporting several international companies in the business of generics in the USA. He is also the Founder and President of AB Pharmaceuticals Inc. He is an active member of the American Pharmaceutical Association as well as a member of the pharmaceutical technical committee in the Slovenian Pharmaceutical Association. He has been a member of the board of directors of the Generic Pharmaceutical Industry Association (GPIA) and member of several other committees of the Generic Pharmaceutical Association (GPhA).

Senior Regulatory Advisor
Rajeev is a technocrat with over 39 years of experience in the Pharmaceutical Industry of which a long stint of 31 years as Head of Global Regulatory Affairs at Lupin. He set a record for getting more than 170 approved ANDAs, guiding numerous successful US FDA inspections, more than 100 Market Authorizations in EU and Australia, prequalification of more than 15 products by WHO, playing significant role in all major regulatory audits of Lupin since 1989. Currently as one of the Directors of PharmSol Group, he is involved in providing business solutions in Pharmaceuticals in the arena of Compliance, Regulatory and Project Management. A postgraduate in Technology of Pharmaceuticals and having extensive experience in the Regulatory Affairs of Drug Products and APIs, Compliance Audits and Product Development (drug product as well as API) for US Markets.

Senior Vice President - Compliance and Regulatory Affairs
Ganesh is Drug Delivery and Formulation Development Expert and provides technical support, design strategies and supporting new development
opportunities in PharmSol. He has more than 25 years of experience in the pharmaceutical drug delivery and dosage form research and development
with an emphasis on process engineering, product development, aseptic manufacturing, and filling. He has working knowledge of all phases from
drug discovery to product commercial manufacturing with expertise in small and large molecule formulations.
He holds a degree in Pharmacy from MGR Medical University, Tamilnadu, Post Graduate degree in Pharmaceutical Sciences from Birla Institute of
Technology (BIT), Ranchi, Doctorate degree in Pharmaceutical Sciences from Maharaja Sayajirao University (MSU), Baroda. EMBA in
Pharmaceutical Technology from Indian Management School and Research Centre (IMSRC), Mumbai.

Senior Scientific Adviser
Ashish is a technocrat with about 40 years of experience in the domain of Pharmaceutical Development, Clinical Research and Clinical Data
Management. He has operational and leadership experience in multiple Clinical Research Organizations including being a founding member of
Lambda Therapeutic Research. He also held leadership position in Pharmalex, a global venture in Regulatory affairs, Medical Writing and
Pharmacovigilance.
Ashish brings an overall experience in multiple service functions of the Pharmaceutical Research, business strategies, mentoring teams, and customer
engagements.
As a part of voluntary service, Ashish is the Management Trustee of Delhi Pharmaceutical Trust, a Trust engaged in consumer awareness on safe use
of medicines.

Vice President, Business Development
Manish, as VP - Business Development, he leads sales and business development for global markets. Manish has over 22 + years of experience in sales, business development and licensing in international business for regulated and emerging markets handling formulations and consumer health business. He has rich exposure in collaborating with cross-cultural clients across a varied geographical layout, showcased excellence in identifying and establishing strategic alliances / tie-ups, market entry strategies and portfolio management.
He holds a Graduation Degree in Chemistry and post graduate diploma in Business Management - Marketing.

Vice President - Quality Affairs
Ravikanth is the Vice President – Quality Affairs of PharmSol Group. In this role, he heads the QA/QC functions, leading the execution team for several technology projects for Formulations and acting as the Chief Inspector of pre-dispatch inspections for API & Formulations. With over 25 years of experience in the areas of Quality Control and Quality Assurance for Bulk Drugs & Formulations, Ravi has also worked across many areas of PharmSol providing support in the 3rd Party GMP Audits and EUGMP Certification projects of PharmSol and acted as Independent Validator towards CEIV Pharma of IATA. He holds a Masters’ Degree in Chemistry and obtained a Diploma in Chemical Processing & Instrumentation Control and Certificates for ECA - Lab Data Integrity, APIC Auditor and CEIV Pharma Independent Validator of IATA

Senior General Manager – Research & Development
Abhay is the Senior General Manager, Projects of PharmSol Group. He is leading the Product and Technology Development functions. He holds a Ph. D. in Pharmaceutical and has been into industry and academics for over 17 years. His professional expertise includes design and development of platform technology, formulation research, process efficiency enhancements, QBD guidelines, scientific community publications and IPR.

Vice President – Manufacturing
Rambabu is the Vice President, looking after the manufacturing, tech transfer and outsourcing functions. He is a M. Pharm in Pharmaceutics and has over 20 years of core experience in pharma production, project management, facility design and upgrade among others.

Director - China, BD
Felix, as Director – China Business Development, leads the business development and growth management functions for PharmSol in China. Prior to joining PharmSol, he has been into diverse roles including business development of CRO, marketing of food & nutraceutical ingredients, among others. He brings with him a strong network of Chinese pharma entrepreneurs. He holds a degree in Information Technology from GD Industry Technical College, China

Director - China, Technical Operations
Nancy has been one of early members of Team PharmSol based out of China and has since grown to become the Director of Technical Operations. She leads a team of compliance and technical experts of PharmSol in China. She has professional expertise in conducting GMP/QP audits, coordinating EU GMP Certifications and project management. Graduated from Shenyang Pharmaceutical University and has about 11+ years of experience in various technical regimes of pharmaceutical industry. She is qualified as an ASQ certified quality Auditor/APIC certified Auditor and Member of European Compliance Academy. As a certified lead auditor, she has successfully performed 200+ audits for various pharmaceutical companies manufacturing OSD, Sterile Dosage formulations and sterile and non-sterile API

Director - China, BD
Shawn has been with PharmSol for 8+ years as part of the Business Development and Commercial Operations in China. As Director – China Business Development, he played a key role in building the local set up of PharmSol in China. With over 15 years of pharmaceutical industry experience with comprehensive expertise in sourcing, import and export of API and intermediates from China, he notably leads the PharmSol Team in dealing with overseas transactions and partnerships on supply chain. Shawn obtained a Master’s Degree in Business Administration from Macau University

Managing Director
Sunil founded PharmSol in 2004 and led the company as Managing Director. His C-level substantial expertise in all facets of Pharmaceutical Business, Diversity Management, International Business, Mergers & Acquisitions and Strategic Alliances brought PharmSol to operational level of excellence and growth. A Chemical Engineer by qualification, with over 36 years of leadership experience in large pharma and established track record of entrepreneurial success within the pharmaceutical industry. Prior to founding PharmSol, he served for 20 years in various MNCs of repute. He has designed and mentored several green field projects for APIs and FDFs, all of which have been USFDA and/or EU certified.

Executive Director
Lakshmi is the Executive Director of PharmSol Group, having responsibility for the company’s strategy function and stakeholder relationships including clients’ relations, and corporate responsibility. He has over 26 years of diversified expertise and operational experience in Regulatory Affairs, QA, IPR, Bulk Drugs, Formulations and Fine Chemical Industries. Prior to his current role, Lakshmi worked in various Indian MNCs and International Companies. Lakshmi is a Pharmaceutical Technologist with Post Graduate Diploma in Patent Law and an MBA in Operations, International Business. His professional accreditations include APIC Certified Auditor, Associate Member of European QP Association, ECA Certified Computer Validation Manager, formerly certified for Temperature - Controlled Cargo Operations and CEIV Pharma Independent Validator of IATA.

Senior Business Associate
Christian has been a Director of PharmSol since inception. He is an entrepreneur with extensive experience in the pharmaceutical & allied fields, has been a guiding brain behind foundation of several businesses in Europe. At PharmSol, he has been supporting the strategy and business development functions. He is a graduate in logistic operations and has transitioned into pharmaceuticals early in his career. His skills in leadership and expertise in assembling people together, while achieving essential aspirations have led to multiple achievements throughout the years.

Director – Strategic Business
Mr. Arun holds a BE (Hons) Degree in Mechanical Engineering from BITS Pilani and a Post Graduate Diploma in Management Studies from JBIMS, University of Mumbai with over 35 years of professional experience. He specializes in international market development in industries related to chemicals, colors, technology, pharmaceuticals, and Agro products. He is also a faculty member in various reputed MBA institutions such as ICT Mumbai, JBIMS, World Trade Institute, and SP Jain Global Management Institute in Mumbai – with a teaching experience of over 22 years. He also works as a consultant to new Indian start-ups in the areas of business building since 2015.

Senior Scientific Adviser
Borut holds a Post Doctorate in molecular biology and has a rich experience of over 35 years into the field of Biosimilar, Nano Technology, Biochemistry and Biotechnology. He was one of the key members of the committee on drafting the EU Guidelines on Biosimilar and was also appointed as an expert in European Pharmacopeia for biological pharmaceuticals as well as at the European Agency for Medicines in London (EMEA). Borut co-authored several patent applications and has written over 180 scientific and professional articles which were published in more than 100 leading scientific journals. Currently, he is heading the Biotechnology Unit and is a full-time professor at the Faculty of Pharmacy of the University of Ljubljana. He is also a part of Editorial Board of the official journal of Slovenian Pharmaceutical Society and International Journal of Medicinal Mushrooms. As a Senior Scientific Adviser of PharmSol, he directs the PharmSol Team that focuses on the development, compliance, and regulatory aspects of biopharmaceutical projects.

Senior Business Associate
Andrej is a US based Pharmacist and Entrepreneur with a range of global experience. He brings to PharmSol a rich global experience of over 25 years within the areas of product development, cross licensing, technology transfers and strategic alliances negotiations and other related operations within the pharmaceutical industry. He holds an Executive MBA degree in Pharmaceutical Marketing and has been supporting several international companies in the business of generics in the USA. He is also the Founder and President of AB Pharmaceuticals Inc. He is an active member of the American Pharmaceutical Association as well as a member of the pharmaceutical technical committee in the Slovenian Pharmaceutical Association. He has been a member of the board of directors of the Generic Pharmaceutical Industry Association (GPIA) and member of several other committees of the Generic Pharmaceutical Association (GPhA).

Senior Regulatory Advisor
Rajeev is a technocrat with over 39 years of experience in the Pharmaceutical Industry of which a long stint of 31 years as Head of Global Regulatory Affairs at Lupin. He set a record for getting more than 170 approved ANDAs, guiding numerous successful US FDA inspections, more than 100 Market Authorizations in EU and Australia, prequalification of more than 15 products by WHO, playing significant role in all major regulatory audits of Lupin since 1989. Currently as one of the Directors of PharmSol Group, he is involved in providing business solutions in Pharmaceuticals in the arena of Compliance, Regulatory and Project Management. A postgraduate in Technology of Pharmaceuticals and having extensive experience in the Regulatory Affairs of Drug Products and APIs, Compliance Audits and Product Development (drug product as well as API) for US Markets.

Senior Vice President - Compliance and Regulatory Affairs
Ganesh is Drug Delivery and Formulation Development Expert and provides technical support, design strategies and supporting new development
opportunities in PharmSol. He has more than 25 years of experience in the pharmaceutical drug delivery and dosage form research and development
with an emphasis on process engineering, product development, aseptic manufacturing, and filling. He has working knowledge of all phases from
drug discovery to product commercial manufacturing with expertise in small and large molecule formulations.
He holds a degree in Pharmacy from MGR Medical University, Tamilnadu, Post Graduate degree in Pharmaceutical Sciences from Birla Institute of
Technology (BIT), Ranchi, Doctorate degree in Pharmaceutical Sciences from Maharaja Sayajirao University (MSU), Baroda. EMBA in
Pharmaceutical Technology from Indian Management School and Research Centre (IMSRC), Mumbai.

Senior Scientific Adviser
Ashish is a technocrat with about 40 years of experience in the domain of Pharmaceutical Development, Clinical Research and Clinical Data
Management. He has operational and leadership experience in multiple Clinical Research Organizations including being a founding member of
Lambda Therapeutic Research. He also held leadership position in Pharmalex, a global venture in Regulatory affairs, Medical Writing and
Pharmacovigilance.
Ashish brings an overall experience in multiple service functions of the Pharmaceutical Research, business strategies, mentoring teams, and customer
engagements.
As a part of voluntary service, Ashish is the Management Trustee of Delhi Pharmaceutical Trust, a Trust engaged in consumer awareness on safe use
of medicines.

Vice President, Business Development
Manish, as VP - Business Development, he leads sales and business development for global markets. Manish has over 22 + years of experience in sales, business development and licensing in international business for regulated and emerging markets handling formulations and consumer health business. He has rich exposure in collaborating with cross-cultural clients across a varied geographical layout, showcased excellence in identifying and establishing strategic alliances / tie-ups, market entry strategies and portfolio management.
He holds a Graduation Degree in Chemistry and post graduate diploma in Business Management - Marketing.

Vice President - Quality Affairs
Ravikanth is the Vice President – Quality Affairs of PharmSol Group. In this role, he heads the QA/QC functions, leading the execution team for several technology projects for Formulations and acting as the Chief Inspector of pre-dispatch inspections for API & Formulations. With over 25 years of experience in the areas of Quality Control and Quality Assurance for Bulk Drugs & Formulations, Ravi has also worked across many areas of PharmSol providing support in the 3rd Party GMP Audits and EUGMP Certification projects of PharmSol and acted as Independent Validator towards CEIV Pharma of IATA. He holds a Masters’ Degree in Chemistry and obtained a Diploma in Chemical Processing & Instrumentation Control and Certificates for ECA - Lab Data Integrity, APIC Auditor and CEIV Pharma Independent Validator of IATA

Senior General Manager – Research & Development
Abhay is the Senior General Manager, Projects of PharmSol Group. He is leading the Product and Technology Development functions. He holds a Ph. D. in Pharmaceutical and has been into industry and academics for over 17 years. His professional expertise includes design and development of platform technology, formulation research, process efficiency enhancements, QBD guidelines, scientific community publications and IPR.

Vice President – Manufacturing
Rambabu is the Vice President, looking after the manufacturing, tech transfer and outsourcing functions. He is a M. Pharm in Pharmaceutics and has over 20 years of core experience in pharma production, project management, facility design and upgrade among others.

Director - China, BD
Felix, as Director – China Business Development, leads the business development and growth management functions for PharmSol in China. Prior to joining PharmSol, he has been into diverse roles including business development of CRO, marketing of food & nutraceutical ingredients, among others. He brings with him a strong network of Chinese pharma entrepreneurs. He holds a degree in Information Technology from GD Industry Technical College, China

Director - China, Technical Operations
Nancy has been one of early members of Team PharmSol based out of China and has since grown to become the Director of Technical Operations. She leads a team of compliance and technical experts of PharmSol in China. She has professional expertise in conducting GMP/QP audits, coordinating EU GMP Certifications and project management. Graduated from Shenyang Pharmaceutical University and has about 11+ years of experience in various technical regimes of pharmaceutical industry. She is qualified as an ASQ certified quality Auditor/APIC certified Auditor and Member of European Compliance Academy. As a certified lead auditor, she has successfully performed 200+ audits for various pharmaceutical companies manufacturing OSD, Sterile Dosage formulations and sterile and non-sterile API

Director - China, BD
Shawn has been with PharmSol for 8+ years as part of the Business Development and Commercial Operations in China. As Director – China Business Development, he played a key role in building the local set up of PharmSol in China. With over 15 years of pharmaceutical industry experience with comprehensive expertise in sourcing, import and export of API and intermediates from China, he notably leads the PharmSol Team in dealing with overseas transactions and partnerships on supply chain. Shawn obtained a Master’s Degree in Business Administration from Macau University

Bad Oldesloe, Germany

Mosta, Malta

Jebel Ali, Dubai, United Arab Emirates

Jumeirah Lake Towers, Dubai

Heliopolis, Cairo, Egypt

Hyderabad, India

Nanjing City, Jiangsu Province, PR China

Hong Kong, China

Makati City, Philippines

Ho Chi Minh City, Vietnam